Publications
• Publication
The Link of the Google Scholar of Professor Hyung-Sool Lee
( https://scholar.google.co.kr/citations?user=1zE6TFsAAAAJ&hl=ko&oi=ao )
As of May 2023, a total of 131 publications
2023
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Production of polyhydroxyalkanoate (PHA) copolymer from food waste using mixed culture for carboxylate production and Pseudomonas putida for PHA synthesis
Abstract
Production of polyhydroxyalkanoates (PHAs) with high concentration of carboxylate, that was accumulated from solid state fermentation (SSF) of food waste (FW), was tested using Pseudomonas putida strain KT2440. Mixed-culture SSF of FW supplied in a high concentration of carboxylate, which caused a high PHA production of 0.56 g PHA/g CDM under nutrients control. Interestingly, this high PHA fraction in CDM was almost constant at 0.55 g PHA/g CDM even under high nutrients concentration (25 mM NH4+), probably due to high reducing power maintained by high carboxylate concentration. PHA characterization indicated that the dominant PHA building block produced was 3-hydroxybutyrate, followed by 3-hydroxy-2-methylvalerate and 3-hydroxyhenxanoate. Carboxylate profiles before and after PHA production suggested that acetate, butyrate, and propionate were the main precursors to PHA via several metabolic pathways. Our result support that mixed culture SSF of FW for high concentration carboxylate and P. putida for PHA production enables sustainable production of PHA in cost-effective manners.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Periodic step polarization accelerates electron recovery by electroactive biofilms (EABs)
Abstract
Relatively low rate of electron recovery is one of the factors that limit the advancement of bioelectrochemical systems. Here, new periodic polarizations were investigated with electroactive biofilms (EABs) enriched from activated sludge and Geobacter sulfurreducens biofilms. When representative anode potentials (Ea) were applied, redox centers with midpoint potentials (Emid) higher than Ea were identified by localized cyclic voltammetry. The electrons held by these redox centers were accessible when Ea was raised to 0.4 V (vs. Ag/AgCl). New periodic polarizations that discharge at 0.4 V recovered electrons faster than normal periodic and fixed-potential polarizations. The best-performing periodic step polarization accelerated electron recovery by 23%−24% and 12%−76% with EABs and G. sulfurreducens biofilms, respectively, compared to the fixed-potential polarization. Quantitative reverse transcription polymerase chain reaction showed an increased abundance of omcZ mRNA transcripts from G. sulfurreducens after periodic step polarization. Therefore, both the rate of energy recovery by EABs and the performance of bioelectrochemical systems can be enhanced by improving the polarization schemes.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Optimization of biofilm conductance measurement with two-electrode microbial electrochemical cells (MECs)
Abstract
This study was conducted to develop a standardized and consistent method for biofilm conductance measurement for an improved comprehension of extracellular electron transfer. Biofilm conductance (2.12 ± 0.25 × 10−4 S) with and without a fixed anode potential did not show significant difference. The conductance showed a sigmoidal relationship with anode potential. The current-voltage profile of the tested biofilm at applied voltage larger than 100 mV showed deviation from Ohm's law. Up to 69% decrease in biofilm conductance and deviation from Ohm's law were observed in the current-voltage profile when the measurement time increased. By choosing the voltage range (0– 100 mV) and step (25 mV), measurement time (100-s at each voltage step), and anode control mode, these operation settings were found more suitable for consistent and accurate biofilm conductance measurement in the 2-Au MEC system. This represents the first study that comprehensively evaluated the environmental and instrumental parameters for biofilm conductance measurement.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

An integrated leachate bed reactor – anaerobic membrane bioreactor system (LBR-AnMBR) for food waste stabilization and biogas recovery
Abstract
This study developed an integrated LBR – AnMBR system for efficient stabilization and biogas recovery from food waste (FW) at room temperatures (21–22 °C). First, the leachate recirculation rate (4.4–13.2 L/h) was optimized to maximize hydrolysis and acidification yields. The maximum hydrolysis yield of 551 gSCOD/kg VSadded was achieved at recirculation rate of 13.2 L/h. The VFA concentrations in the FW leachate was as high as 12.5–16.0 g/L, resulting in a high acidification of 468 g CODVFA/kg VS. The solubilized FW was further stabilized by feeding the leachate to AnMBR. Different hydraulic (HRT) and solids retention times (SRT) were tested to achieve high COD removal and methane yields. High COD removal of 86 ± 3% was obtained in the AnMBR at HRT of 13 and SRT of 75 days. High biogas recovery of about 850 kWh per ton FWtreated was achieved along with high quality of AnMBR permeates containing low COD concentration but advantageously high concentration of nutrients (NH4+-N 317–403 mg/L, total phosphate 23–213 mg/L) without any particulates, which can be reused for landscape or liquid fertilizer.
2022
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Biohydrogen production and purification: Focusing on bioelectrochemical systems
Abstract
Innovative technologies on green hydrogen production become significant as the hydrogen economy has grown globally. Biohydrogen is one of green hydrogen production methods, and microbial electrochemical cells (MECs) can be key to biohydrogen provision. However, MECs are immature for biohydrogen technology due to several limitations including extracellular electron transfer (EET) engineering. Fundamental understanding of EET also needs more works to accelerate MEC commercialization. Interestingly, studies on biohydrogen gas purification are limited although biohydrogen gas mixture requires complex purification for use. To facilitate an MEC-based biohydrogen technology as the green hydrogen supply this review discussed EET kinetics, engineering of EET and direct interspecies electron transfer associated with hydrogen yield and the application of advanced molecular biology for improving EET kinetics. Finally, this article reviewed biohydrogen purification technologies to better understand purification and use appropriate for biohydrogen, focusing on membrane separation.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Acidogenic fermentation of food waste in a leachate bed reactor (LBR) at high volumetric organic Loading: Effect of granular activated carbon (GAC) and sequential enrichment of inoculum
Abstract
This study investigated the impact of different granular activated carbon (GAC) loadings and inoculum enrichment on acidogenic fermentation of food waste in a leachate bed reactor (LBR) operated at a high volumetric organic loading of 49 g VS/Lreactor. LBR with a high GAC loading of 0.51 g GAC/g VSfood waste achieved hydrolysis yield of 620 g SCOD/kg VSadded, significantly (P ≤ 0.05) higher to that obtained for LBRs with low or no GAC loading. A high GAC loading also resulted in a higher acidification yield of 507 g CODSCFA/kg VSadded. Butyrate dominated the short-chain fatty acid (SCFA) composition by constituting 57–60 % of total SCFA at high GAC loadings, while the composition of acetate (38–40 %) and butyrate (36–38 %) were similar at lower GAC loadings. Inoculum enrichment further improved the hydrolysis and acidogenesis yields by 10–22 % resulting in the final hydrolysis yield of 683 g SCOD/kg VSadded and acidification yield of 617 g CODSCFA/kg VSadded.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Nitrite reduction using a membrane biofilm reactor (MBfR) in a hypoxic environment with dilute methane under low pressures
Abstract
Methane-based membrane biofilm reactors (MBfRs) can be an effective solution for nitrogen control in wastewater, but there is limited information on nitrite reduction for dilute wastewater (e.g., municipal wastewater) in hypoxic MBfRs. This study assessed the impacts of dilute (20 %), low-pressure methane (0.35–2.41 kPa) applied to MBfRs at hydraulic retention times (HRTs) of 2–12 h on nitrite removals, dissolved methane concentrations, and the resulting changes in the microbial community. High nitrite flux along with rapid and virtually complete (>99 %) nitrite removals were observed at methane pressures of 1.03–2.41 kPa at HRTs above 4 h, despite the use of diluted methane gas for the MBfR. The lowest methane pressure (0.35 kPa) was also able to achieve up to 98 % nitrite removals but required HRTs of up to 12 h. All scenarios had low dissolved methane concentrations (<10 mg/L), indicating that dilute methane at low supply pressures can effectively remove nitrite while meeting dissolved methane guidelines in treated effluent. Methylococcus genus was the key bacterium in MBfR biofilm grown at different HRTs and methane pressures, along with Methylocystis and other heterotrophic denitrifiers (Terrimonas and Hyphomicrobium). This study indicates that methane-based denitrification MBfRs can be a valuable tool to meet nitrogen limits for dilute wastewater coupled to partial nitrification, while limiting the release of methane to the environment.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Microbial electrolysis cells for the production of biohydrogen in dark fermentation – A review
Abstract
To assess biohydrogen for future green energy, this review revisited dark fermentation and microbial electrolysis cells (MECs). Hydrogen evolution rate in mesophilic dark fermentation is as high as 192 m3 H2/m3-d, however hydrogen yield is limited. MECs are ideal for improving hydrogen yield from carboxylate accumulated from dark fermentation, whereas hydrogen production rate is too slow in MECs. Hence, improving anode kinetic is very important for realizing MEC biohydrogen. Intracellular electron transfer (IET) and extracellular electron transfer (EET) can limit current density in MECs, which is proportional to hydrogen evolution rate. EET does not limit current density once electrically conductive biofilms are formed on anodes, potentially producing 300 A/m2. Hence, IET kinetics mainly govern current density in MECs. Among parameters associated with IET kinetic, population of anode-respiring bacteria in anode biofilms, biofilm density of active microorganisms, biofilm thickness, and alkalinity are critical for current density.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

The role of microbial electrogenesis in regulating methane and nitrous oxide emissions from constructed wetland-microbial fuel cell
Abstract
This study assesses a sustainable solution to greenhouse gases (GHGs) mitigation using constructed wetland-microbial fuel cells (CW-MFC). Roots of wetland plant Acorus Calamus L. are placed in biological anode to better enable anode microorganisms to obtain rhizosphere secretion for power improvement. Three selected cathode materials have a large difference in GHG emissions, and among them, carbon fiber felt (CFF) shows the lowest emissions of methane and nitrous oxide, which are 0.77 ± 0.04 mg/(m2·h) and 130.78 ± 13.08 μg/(m2·h), respectively. The CFF CW-MFC achieves the maximum power density of 2.99 W/m3. As the influent pH value is adjusted from acidic to alkaline, the GHGs emissions are reduced. The addition of Ni inhibits GHGs emission but decreases the electricity, the power density is reduced to 1.09 W/m3, and the methane and nitrous oxide emission fluxes decline to 0.20 ± 0.04 mg/(m2·h) and 15.49 ± 1.86 μg/(m2·h), respectively. Low C/N ratio reduces methane emission, while high C/N ratio effectively inhibits nitrous oxide emission. At the influent pH 8 and C/N = 5:1, the methane emission flux is approximately 10.60 ± 0.27 mg/(m2·h), and the nitrous oxide emission flux is only 10.90 ± 1.10 μg/(m2·h). Based on the above experimental results by controlling variable factors, it is proposed that CW-MFC offers an environment-friendly solution to regulate GHG emissions.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
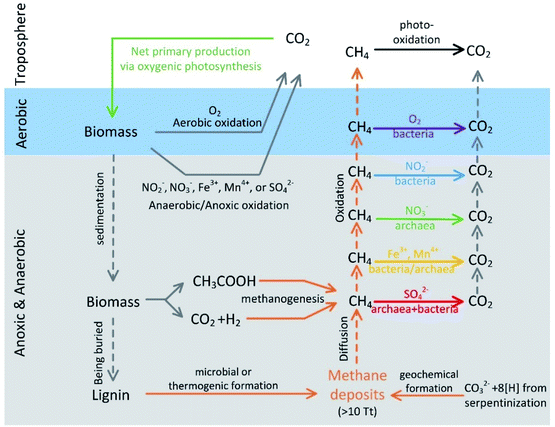
Significance of anaerobic oxidation of methane (AOM) in mitigating methane emission from major natural and anthropogenic sources: a review of AOM rates in recent publications
Abstract
Methane is estimated to have contributed 20% of postindustrial global warming. Methanotrophs oxidize methane and curb methane emissions into the atmosphere. Anaerobic oxidation of methane (AOM) has been recognized as an important methane sink. Sulfate is the primary electron acceptor of AOM in the marine environment, while nitrite/nitrate is encountered more often in terrestrial water-logged systems, such as rice paddy and wetlands. A key aspect of AOM is the reaction rate, which influences methane fluxes to the oxic zones and eventually the atmosphere. We collated the AOM rates from major natural and anthropogenic sources in recent publications and found that AOM rates are generally lower than the corresponding aerobic methane oxidation rates in wetlands and rice paddy, while the AOM rates are often higher than the corresponding aerobic oxidation rates in freshwater systems and marine environments. Based on the median reaction rates and estimated aerobic and anoxic zone coverages, AOM consumes approximately 71%, 8%, 5%, 13%, and 3% of the methane entering the anoxic zones in oceans, wetlands, paddy systems, lakes/reservoirs, rivers, respectively. These analyses suggest that AOM is a key methane sink in oceans, while aerobic methanotrophs consume more methane in the other studied ecosystems. Finally, the controlling factors of AOM and some issues in the rate quantification were discussed. It is believed that more comprehensive studies of AOM and improved rate quantification would assist in forecasting methane emission, which fosters scientific debate over global warming and eventually affects climate policymaking.
2021
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Butyrate production and purification by combining dry fermentation of food waste with a microbial fuel cell
Abstract
This study developed and evaluated a high-purity butyrate producing bioprocess from food waste by combining dry fermentation (DF) with a microbial fuel cell (MFC). Acclimatization of a DF reactor with an enrichment culture resulted in high food waste degradation (VS removed, %) and butyrate production. A high VS degradation of 81%, butyrate concentration of up to 24 gCODbutyrate/L and butyrate yields of 497 gCODbutyrate/kg VSadded was obtained in the DF reactor. As a result, butyrate comprised 83% of all short chain fatty acids (SCFA) in the DF broth. Acetate (10%) and propionate (7%) comprised the rest of the SCFA. The butyrate composition was further purified by feeding the DF broth to a multi-electrode MFC enriched with anode respiring bacteria (ARB) such as Geobacter sp. (>55%). The ARB in the MFC removed acetate and propionate while purified butyrate was recovered in the MFC effluent. Butyrate purity in the MFC effluent reached as high as 99% at hydraulic retention time of 72 h. Along with butyrate purification, the MFC produced electric power in a range of 0.1–0.6 Wh/gCODbutyraterecovered (or 0.01–7.85 kWh/ton of food waste), demonstrating that MFCs can be an energy-positive butyrate purification bioprocess.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

State-of-the-art management technologies of dissolved methane in anaerobically-treated low-strength wastewaters: A review
Abstract
The recent advancement in low temperature anaerobic processes shows a great promise for realizing low-energy-cost, sustainable mainstream wastewater treatment. However, the considerable loss of the dissolved methane from anaerobically-treated low-strength wastewater significantly compromises the energy potential of the anaerobic processes and poses an environmental risk. In this review, the promises and challenges of existing and emerging technologies for dissolved methane management are examined: its removal, recovery, and on-site reuse. It begins by describing the working principles of gas-stripping and biological oxidation for methane removal, membrane contactors and vacuum degassers for methane recovery, and on-site biological conversion of dissolved methane into electricity or value-added biochemicals as direct energy sources or energy-compensating substances. A comparative assessment of these technologies in the three categories is presented based on methane treating efficiency, energy-production potential, applicability, and scalability. Finally, current research needs and future perspectives are highlighted to advance the future development of an economically and technically sustainable methane-management technology.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

A modelling study of the spatially heterogeneous mutualism between electroactive biofilm and planktonic bacteria
Abstract
Microbial cooperation widely exists in anaerobic reactors degrading complex pollutants, conventionally studied separately inside the biofilm or the planktonic community. Recent experiments discovered the mutualism between the planktonic bacteria and electroactive biofilm treating propionate, an end-product usually accumulated in anaerobic digesters. Here, a one-dimensional multispecies model found the preference on acetate-based pathway over the hydrogen-based in such community, evidenced by the fact that acetate-originated current takes 66% of the total value and acetate-consuming anode-respiring bacteria takes over 80% of the biofilm. Acetate-based anodic respiration most apparently influences biofilm function while propionate fermentation is the dominant planktonic bio-reaction. Additionally, initial planktonic propionate level shows the ability of coordinating the balance between these two extracellular electron transfer pathways. Increasing the propionate concentration from 2 to 50 mM would increase the steady hydrogen-originated current by 210% but decrease the acetate-originated by 26%, suggesting a vital influence of the planktonic microbial process to the metabolic balance in biofilm. Best strategy to promote the biofilm activity is to increase the biomass density and biofilm conductivity simultaneously, which would increase the current density by 875% without thickening the biofilm thickness or prolonging the growth apparently.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Anaerobic membrane bioreactors for wastewater treatment: Challenges and opportunities
Abstract
Anaerobic membrane bioreactors (AnMBRs) have become a new mature technology and entered into the wastewater market, but there are several challenges to be addressed for wide applications. In this review, we discuss challenges and potentials of AnMBRs focusing on wastewater treatment. Nitrogen and dissolved methane control, membrane fouling and its control, and membrane associated cost including energy consumption are main bottlenecks to facilitating AnMBR application in wastewater treatment. Accumulation of dissolved methane in AnMBR permeate decreases the benefit of methane energy and contributes to methane gas emissions to atmosphere. Separate control units for nitrogen and dissolved methane add system complexity and increase capital and operating and maintenance (O & M) costs in AnMBR-centered wastewater treatment. Alternatively, methane-based denitrification can be an ideal nitrogen control process due to simultaneous removal of nitrogen and dissolved methane. Membrane fouling and energy associated with membrane fouling control are major limitations, in addition to membrane cost. More efforts are required to decrease capital and O & M costs associated with the control of dissolved methane nitrogen and membrane fouling to facilitate AnMBRs for wastewater treatment.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

A quantitative extracellular electron transfer (EET) kinetics study of Geobacter sulfurreducens enriched microbial community reveals the transition of EET limiting step during biofilm growth
Abstract
Extracellular electron transfer (EET) allows exoelectrogens to transfer electrons outside their outer membrane as a unique process to complete respiration. The EET kinetics of Geobacter sulfurreducens is critical to further improving the performance of microbial electrochemical technologies such as microbial fuel cells. However, the EET of Geobacter sulfurreducens is not yet fully understood, and current and power densities of microbial fuel cells meet with a bottleneck. This work addresses this deficit by studying the kinetic parameters of the EET of Geobacter sulfurreducens enriched microbial community for a series of biofilm growth stages through the non-linear fitting of discharging current profiles of micro-scale microbial fuel cells. The quantitative EET rate constants and the amount of redox cofactors associated with individual EET steps are obtained from initial to fully-grown biofilms. This quantitative study reports the rate-limiting step in EET transitions during biofilm growth. In early to mid-stage biofilms, we report current densities of less than 2.2 Am−2 and between 2.2 and 3.1 Am−2, respectively. The rate-limiting step transitions from irreversible acetate turnover to the electron transfer from inside the exoelectrogen to extracellular redox cofactors (ERCs) within the biofilm. We show fully-grown biofilms have a current density of more than 3.1 Am−2, and the rate-limiting step here is instead the electron transfer from ERCs within the biofilm to ERCs at the anode. The results of this study illuminate the mechanisms of the EET of Geobacter sulfurreducens, using quantitative reaction kinetics parameter analysis.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Examination of Extracellular Polymer (EPS) Extraction Methods for Anaerobic Membrane Bioreactor (AnMBR) Biomass
Abstract
Membrane bioreactor fouling is a complex process, which is typically driven by extracellular polymeric substances (EPS), a complex mixture of polysaccharides, proteins, lipids, humic substances, and other intercellular polymers. While much is known about fouling in aerobic membrane reactors, far less is known about fouling in anaerobic membrane bioreactors (AnMBR). Much of this knowledge, including EPS extraction methods, has been extrapolated from aerobic processes and is commonly assumed to be comparable. Therefore, several extraction methods commonly used for aerobic EPS quantification, including ultrasonication, ethylenediaminetetraacetic acid (EDTA), and formaldehyde plus sodium hydroxide (CH2O+NaOH), were evaluated to determine the most suitable extraction method for EPS of anaerobic microorganisms in an AnMBR. To maximize EPS yields, each extraction was performed four times. Experimental results showed that the EDTA method was best for EPS quantification, based on chemical oxygen demand (COD), dissolved organic carbon (DOC), and protein yields: 1.43 mg COD/mg volatile suspended solids (VSS), 0.14 mg DOC/mg VSS, and 0.11 mg proteins/mg VSS. In comparison, the CH2O+NaOH method maximized the extraction of carbohydrates (0.12 mg carbohydrates/mg VSS). However, multiple extraction cycles with EDTA and ultrasonication exhibited lower extracellular adenosine triphosphate (ATP) concentrations compared to CH2O+NaOH extractions, indicating lower levels of released intracellular substances. Successive EPS extractions over four cycles are better able to quantify EPS from anaerobic microorganisms, since a single extraction may not accurately reflect the true levels of EPS contents in AnMBRs, and possibly in other anaerobic processes.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Spatial distribution of biofilm conductivity in a Geobacter enriched anodic biofilm
Abstract
This study assessed the spatial distribution of biofilm conductivity (Kbio) across a multi-centimetre long anodic biofilm grown on an eight-electrode anode array at different growth conditions. A strong correlation was found between the spatial distribution of Kbio and the anodic biofilm thickness (Lf). The Kbio for different electrode pairs ranged between 0.6 and 0.7 mS/cm for Lf of 17–22 µm and increased to 1.15–1.64 mS/cm upon Lf growth to 38–53 µm. This increase in Kbio was accompanied by increase in current density from 1.15 ± 0.12 A/m2 to 2.1 ± 0.02 A/m2. Low half saturation potential was consistently found for electrode pairs having high values of Kbio and Lf. Microbial community revealed the dominance of Geobacter (>85%) on all electrode pairs. Acetate concentration significantly influenced the spatial distribution of Kbio. Long-term acetate starvation (3 days) resulted in up to 83% drop in Kbio along with decrease in current density to marginal values (<0.3 A/m2). However, the Kbio and current density rapidly recovered on restoring the acetate feed.
2020
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

The micro-niche of exoelectrogens influences bioelectricity generation in bioelectrochemical systems
Abstract
Bioelectrochemical systems (BESs) employ exoelectrogens to degrade organic matter, and produce value-added products, such as bioelectricity, methane, acetate and hydrogen. Based on the advantages of energy saving and resource recovery, BES is expected to play an important role in sustainable wastewater treatment in the future. Although considerable progress has been made in the past two decades, the commercialization of BES still needs to overcome many technical challenges to reduce costs and increase electricity output. A comprehensive understanding of the working mechanism of exoelectrogens rooted in the defined micro-niche that covers interactions with electrolytes, electrodes and other microorganisms, is the premise of solving the problem. To provide theoretical guidance for BES performance optimization, this review summarized the defined micro-niche of exoelectrogens in BES, including the overall set environmental conditions of BES and the local microenvironment of electroactive biofilms (EABs). With the expansion of EAB, the microenvironment of EAB slightly shifts in pH gradient, metabolic activity, electron transfer pathways and synergetic growth with other microorganisms. Exoelectrogens in different niche have different contributions to current, and this can be adjusted by optimization of substrate, electrode material, electrode potential, etc. In summary, an efficient and stable micro-niche is the key to ensure that BES continues to produce bioelectricity and remove pollutants, providing guidance for BES design and operation in the future.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Valorization of food waste and economical treatment: Effect of inoculation methods
Abstract
We evaluated cost-effective food waste (FW) treatment and synthesis of volatile fatty acids (VFA) using a leach bed reactor (LBR) operated at neutral pH and room temperature to maximize the profit from recovered products. Three inoculation strategies that include (1) inoculum pretreatment, (2) pretreatment plus leachate recycle, and (3) sole leachate recycle were examined and LBR performance was compared in these inoculation conditions. The inoculation method influenced the hydrolysis of FW in the LBR. Hydrolysis yield was as high as 771 ± 4 g cum.sCOD/kg VSadded with the second inoculation method only in a short reaction time of 6 d. Butyric and acetic acids consistently dominated VFA, and the LBR achieved the highest VFA yield of 649 ± 13 g COD/kg VSadded in the second inoculation. Bacterial community dynamics targeting 16S rRNA gene suggested that Roseburia would be the key player to fermentation of slowly biodegradable FW (e.g., fibers), while multiple types of bacteria affiliated with Enterobacteriaceae, Lactococcus, and Bacteroides mainly fermented FW to butyric and acetic acids at initial phase. The operating cost for FW treatment was calculated $88.1-$126.8 per ton of VSadded in the LBR. This study suggests that LBRs can economically stabilize FW only in 6 d without regular inoculation of exogenous microorganisms, along with high VFA recovery.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Recovery of dissolved methane from anaerobically treated food waste leachate using solvent-based membrane contactor
Abstract
The difficulty of dissolved methane recovery remains a major hurdle for mainstream anaerobic wastewater treatment processes. We recently proposed solvent-based membrane contactor (SMC) for high (>90%) methane recovery over a wide temperature range and net-energy production. Here, we investigate the methane recovery efficacy of the SMC process by using an AnMBR effluent from treating food waste leachate. We observed almost identical methane transfer kinetics to the process employing foulant-free methane-saturated feed solutions, with >92% methane recoveries, showing that organic foulants have insignificant impacts on the methane transport in the SMC. We then performed two different membrane contactor experiments: direct-contact membrane-distillation (DCMD, with transmembrane water vapor flow) and SMC (no water vapor flow). From the negligible fouling observed in the SMC experiment, opposite to the DCMD, we elucidate that the absence of water vapor flow renders the SMC process intrinsically robust to membrane fouling. With the low fouling propensity of the SMC process under highly fouling environments, our study highlights the feasibility of SMC processes to enhance the energy production in mainstream anaerobic wastewater treatment processes.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Hydrophilic graphene aerogel anodes enhance the performance of microbial electrochemical systems
Abstract
The hydrophilic three-dimensional (3D) structure of graphene materials was produced with reducing agent-ethylene glycol through hydrothermal reduction. Numerous microorganisms with diverse community structure were established in anode surface, as the hydrophilicity of the graphene anode increased; more populations of Proteobacteria and Firmicutes families were identified in a higher hydrophilic anode. In addition, the start-up time of a microbial fuel cell (MFC) equipped with hydrophilic 3D graphene anode was only 43 h, which is much shorter than traditional 3D graphene-based anode systems. The most hydrophilic anode exhibited the maximal power density of 583.8 W m−3, 5 times larger than the least hydrophilic one. The content of oxygen in graphene materials improving hydrophilicity would play an important role in enhancing power density. This study proves that hydrophilic 3D graphene materials as the anode can improve MFC performance and start-up time.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

High-rate carboxylate production in dry fermentation of food waste at room temperature
Abstract
Dry fermentation of food waste was optimized to achieve the maximum solid loading rate for carboxylate production without clogging events in a dry fermenter run at neutral pH. High inoculum-to-substrate ratio improved food waste solubilization and carboxylate yield, but the ratio 15% completely clogged the dry fermenter. Higher leachate circulate rate tended to enhance food waste fermentation, but partial clogging was observed at 13.2 L/h of leachate circulation rate. The dry fermenter achieved carboxylate yield of 428.5 g/kg food waste and volatile solid reduction of 79% at the solid loading rate 4.82 kg volatile solids/m3-d. This study first tracks chemical oxygen demand (COD) in food waste dry fermentation, showing maximum soluble COD <60% of food waste COD with residual food waste 13.6–16.3%. The operating cost was as low as $1.7/ton FW, implying that food waste treatment will be cost-neutral if recovered carboxylate can create economic benefit over the operating cost.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Efficient hydrogen recovery with CoP-NF as cathode in microbial electrolysis cells
Abstract
The emerging microbial electrolysis cell (MEC) is of great potential for energy recovery from wastewater as hydrogen, while the application of MECs required cost-effective, scalable cathodes. Here, the phosphating cobalt (CoP) acicular nanoarray in-situ growing on a 3D commercial nickel foam (NF) matrix without binder demonstrated outstanding electrocatalytic activity. The nano-CoP coated NF (CoP-NF) was assessed for hydrogen recovery performance in MEC. In electrochemical testing, the CoP-NF demonstrated comparable performance to a commercial Pt/C catalyst in linear scan voltammetry tests. The lower Tafel slope of 175.4 mV dec−1 in 1 M phosphate buffer electrolyte indicated more favorable electrochemical kinetics of CoP-NF than commercial Pt/C. The CoP-NF demonstrated higher electrochemical active surface area of electrode-liquid interface for its acicular nano-structure, while the smaller charge transfer resistance of CoP-NF suggested the faster electron transfer rate and catalytic activity of hydrogen evolution reaction. An improved hydrogen production rate of 222 ± 20.3 mL H2 L−1 d−1 at 0.7 V applied voltage in fully assembled MECs was achieved for the outstanding hydrogen evolution performance of CoP-NF cathode, which was 3-fold superior to bare NF and even better than the Pt/C. The energy efficiency based on input electricity in the MECs equipped with the CoP-NF (90 ± 6.5%) was increased to twice as much as that Pt/C based cathodes. Long-term operation tests of MECs confirmed the superior stability of CoP-NF as an effective cathode in MECs for hydrogen production, suggesting its potential possibility in practical application.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Mixed dye wastewater treatment in a bioelectrochemical system-centered process
Abstract
The feasibility of mixed dye wastewater treatment was evaluated with a novel integrated bioprocess that consisted of a hybrid anaerobic reactor (HAR) with a built-in bioelectrochemical system, an aerobic biofilm reactor (ABFR) and a denitrification reactor (DR). The position of the DR significantly affected chemical oxygen demand (COD) and colority in effluent, and placing the DR after the ABFR improved effluent quality probably due to minimization of the undesired autoxidation of aromatic amine in dye wastewater. The optimal integrated process of HAR + ABFR + DR efficiently treated mixed dye wastewater, and concentrations of COD and TN were decreased down to 75 ± 18 mg/L and 12.91 ± 0.31 mg/L, respectively, along with colority 48 ± 4 times. Total phosphorus reduced to below 0.5 mg/L with coagulation using poly aluminum chloride, and the effluent quality fully met the discharge standard. This comprehensive study suggests the feasibility of the BES based process for practical application to mixed dye wastewater treatment.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Understanding the Significance of Current Density in Microbial Electrochemical Cells
Abstract
Current microbial electrochemical cells (MxCs) do not meet requirements of anaerobic waste and wastewater treatment, mainly due to sluggish kinetics and small current density. Hence, improving current density is essential to deploy MxCs in the field as energy-efficient anaerobic wastewater treatment. To better understand the limiting parameters and operating conditions associated with current density, this review mechanistically analyzed the biological kinetic parameters in biofilm anodes in parallel with operating conditions related to the parameters. Six biological parameters that include electron fraction used for catabolism ( f e o https://s3-euw1-ap-pe-df-pch-content-public-p.s3.eu-west-1.amazonaws.com/9780429487118/b8b3b135-94fb-4bd6-a54f-c8a61850878b/content/eq130.tif"/> ), apparent maximum specific substrate-utilization rate (qmax,app), biofilm density of active exoelectrogens (Xf), biofilm thickness (Lf), concentration of donor substrate (Sd) and apparent half-saturation concentration (Ksd,app) can influence current density in a biofilm anode given that extracellular electron transfer (EET) would not limit current density. The proliferation of exoelectrogens can increase f e o https://s3-euw1-ap-pe-df-pch-content-public-p.s3.eu-west-1.amazonaws.com/9780429487118/b8b3b135-94fb-4bd6-a54f-c8a61850878b/content/eq131.tif"/> and Xf, but all exoelectrogens do not have high kinetic features, thus, indicating the importance of enriching kinetically efficient exoelectrogens (i.e., Geobacter), not just exoelectrogens. Thick Lf cannot simply increase current density due to acidification of inner biofilms in thick biofilms related to Xf term. It is detrimental to optimize qmax,app, Ksd,app, Xf and Lf in biofilm anodes to given wastewater to achieve current density required for field application along with engineering approach.
2019
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Methane-based denitrification kinetics and syntrophy in a membrane biofilm reactor at low methane pressure
Abstract
A methane-based membrane biofilm reactor (MBfR) was assessed for a tertiary nitrogen removal process in domestic wastewater treatment. To mitigate effluent dissolved methane concentrations, the MBfR was operated with a 20% methane mixing ratio and a low pressure of 0.003 atm. The nitrate concentration was reduced from 20 to 4 mg/L with a low methane concentration of 3.3 mg/L in the effluent at 4 h hydraulic retention time (HRT). An in situ dissolved oxygen sensor showed a concentration of 0.045 mg/L in the MBfR, demonstrating methane oxidation under hypoxic conditions. Both 16S rRNA gene sequencing and metagenomic analysis identified bacteria capable of oxidation of methane coupled to denitrification (Methylocystis), whereas other bacteria were implicated in either methane oxidation (Methylococcus) or nitrate reduction (Escherichia). Reduced genetic potential for nitrate reduction to nitrite at a shorter HRT coincided with a decreased efficiency of denitrification, suggesting rate limitation by this initial step of denitrification. Genes encoding nitrite reduction to dinitrogen were at similar relative abundance under both HRT conditions. Our results provide mechanistic evidence for microbial syntrophy between aerobic methanotrophs and denitrifiers in methane-fed MBfRs operated under varying HRTs, with important implications for novel biological nitrogen removal to dilute wastewater.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Extraction of dissolved methane from aqueous solutions by membranes: Modelling and parametric studies
Abstract
A parametric study was carried out for the extraction of dissolved methane from aqueous solutions using membranes. Both nonporous membranes based on rubbery polymers (e.g., polydimethylsiloxane (PDMS) and poly(ether-b-amide) (PEBA)) and hydrophobic microporous membranes (e.g., polytetrafluoroethylene (PTFE)) were evaluated. Through modelling and parametric analyses, the effects of concentration polarization in the liquid phase at the feed side on extraction of dissolved methane from aqueous solutions were evaluated. It was shown that when the concentration polarization in the liquid boundary layer was negligible, the microporous PTFE membrane outperformed the nonporous membranes in terms of methane flux (3–5 orders of magnitude higher than the nonporous membranes) and methane concentration in the wet permeate stream (~97 mol.% with the PTFE membrane). However, under normal hydrodynamic conditions, the effect of concentration polarization at the feed side was found not to be negligible, and the methane extraction flux was compromised significantly (with a flux reduction of 90% for PTFE and PDMS membranes and 50% for PEBA membrane, at a permeate pressure of 10 kPa). It was found that increasing the permeate pressure would mitigate concentration polarization at the expense of lowered flux. Proper control of the hydrodynamic conditions of feed liquid to enhance mass transfer near the membrane surface appears to be a practical approach to the degassing application.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Kinetics of anaerobic methane oxidation coupled to denitrification in the membrane biofilm reactor
Abstract
Anaerobic oxidation of methane coupled to denitrification (AOM-D) in a membrane biofilm reactor (MBfR), a platform used for efficiently coupling gas delivery and biofilm development, has attracted attention in recent years due to the low cost and high availability of methane. However, experimental studies have shown that the nitrate-removal flux in the CH4-based MBfR (<1.0 g N/m2-day) is about one order of magnitude smaller than that in the H2-based MBfR (1.1–6.7 g N/m2-day). A one-dimensional multispecies biofilm model predicts that the nitrate-removal flux in the CH4-based MBfR is limited to <1.7 g N/m2-day, consistent with the experimental studies reported in the literature. The model also determines the two major limiting factors for the nitrate-removal flux: The methane half-maximum-rate concentration (K2) and the specific maximum methane utilization rate of the AOM-D syntrophic consortium (kmax2), with kmax2 being more important. Model simulations show that increasing kmax2 to >3 g chemical oxygen demand (COD)/g cell-day (from its current 1.8 g COD/g cell-day) and developing a new membrane with doubled methane-delivery capacity (Dm) could bring the nitrate-removal flux to ≥4.0 g N/m2-day, which is close to the nitrate-removal flux for the H2-based MBfR. Further increase of the maximum nitrate-removal flux can be achieved when Dm and kmax2 increase together.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Food waste treatment with a leachate bed reactor: Effects of inoculum to substrate ratio and reactor design
Abstract
This study evaluated the effects of different inoculum to substrate ratios (ISRs) (5, 10, 15%) on hydrolysis and acidogenesis of food waste in a conventional leachate bed reactor (LBR-C) and a novel fractionalized LBR (LBR-F). At ISR of 10%, LBR-C experienced clogging and thus the solid removal and VFA production reduced significantly. Without any clogging events at high ISR of 10%, LBR-F achieved significantly higher (p < 0.05) VS removal of 91%, hydrolysis yield of 837 g cumulative sCOD/kg volatile solids (VS), and VFA yield of 669 g COD/kg VS. Hydrogen yield was as high as 20 m3/ton food waste in LBR-F. Energy balance indicated that the LBR-F can be energy-positive for food waste treatment with net energy benefit of ∼8 kWh/ton food waste treated. Considering the high VFA yield, the LBR-F can also be a promising food waste fermentation system for the biorefinery platform.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Induction of cathodic voltage reversal and hydrogen peroxide synthesis in a serially stacked microbial fuel cell
Abstract
We developed an innovative strategy to address the inhibition of anode-respiring bacteria due to voltage reversal in serially stacked microbial fuel cells by inducing cathodic voltage reversal and H2O2 production. When platinum-coated carbon (Pt/C) cathodes were employed (stacked MFCPt/C) and the MFC was operated with acetate medium, the last unit (MFC 4) caused a voltage reversal of −0.8 V with a substantial anode overpotential of 1.22 V. After replacing the Pt/C cathode with a Pt-free carbon gas diffusion electrode in MFC 4, an electrode overpotential, approximately 0.5 V, was shifted from the anode to the cathode, inducing cathodic voltage reversal. Under cathodic voltage reversal, MFC 4 generated H2O2 at a production rate of 117 mg H2O2/m2-h. Hence, under cathodic voltage reversal induced by Pt-free cathodes, due to less anode polarization, the anode-respiring activity can largely be sustained in a stacked MFC that treats organic wastewater consistently and the quality of treated wastewater may be improved with energy-efficient and on-site generated H2O2.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery
Abstract
In recent years, ever-increasing socio-economic awareness, and negative impact of excessive petro consumption have redirected the research interests towards bio-resources such as algal-based biomass. In order to meet current bio-economy challenges to produce high-value multiple products at a time, new integrated processes in research and development are necessary. Though various strategies have been posited for conversion of algal-based biomass to fuel and fine chemicals, none of them has been proved as economically viable and energetically feasible. Therefore, a range of other bio-products needs to be pursued. In this context, the algal bio-refinery concept has appeared with notable solution to recover multiple products from a single operation process. Herein, an algal-based bio-refinery platform for fuel, food, and pharmaceuticals considering Bio-refinery Complexity Index (BCI) has been evaluated, as an indicator of techno-economic risks. This review presents recent developments on algal-biomass utilization for various value-added products as part of an integrated bio-refinery.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Hydrogen-based syntrophy in an electrically conductive biofilm anode
Abstract
We experimentally and theoretically investigated implications of H2 and a rate-limiting step in a mixed-culture biofilm anode fed with n-butyrate, one of the poorest substrates to exoelectrogens. Acetate and i-butyrate were formed as intermediates during anaerobic degradation of n-butyrate, which suggested oxidative acetogenesis of n-butyrate in syntrophy with H2 scavengers in the biofilm anode. Methane was not detected in an anode chamber, and no current was generated in the biofilm anode using H2 as the electron donor. These results indicated that acetogens would be a main H2 consumer in the biofilm. Pyrosequencing data showed dominance of Geobacter in the biofilm anode (83.6% of total sequences), along with Sphaerochaeta and Treponema, which supports the syntrophy between exoelectrogens and acetogens. Electrical conductivity of the butyrate-fed biofilm anode was as high as 0.67 mS/cm, demonstrating that EET does not limit current density in the biofilm. In-situ monitoring of dissolved H2 concentration proved H2 production (up to 12.4 µM) and consumption during current generation in the biofilm, which supports significance of H2–based syntrophy in the electrically conductive biofilm using n-butyrate as the primary electron donor.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Food waste fermentation in a leach bed reactor: Reactor performance, and microbial ecology and dynamics
Abstract
Food waste fermentation was investigated in a leach bed reactor operated at acidic, neutral and alkaline conditions. Highest solids reduction of 87% was obtained at pH 7 in 14 days of reaction time with minimum mixing. The concentration of volatile fatty acids increased to 28.6 g COD/L under pH 7, while the highest butyric acid of 16 g COD/L was obtained at pH 6. Bacterial community structure was narrowed down to Bifidobacterium and Clostridium at pH 6, while Bacteroides and Dysgonomonas were identified as main players at both pH 7 and 8. Bacterial populations in the food residue generally reflected those in the leachate, but some bacteria were selectively enriched in the leachate or the food residue. Bacterial community dynamics suggested that biodegradable food waste was first fermented by one of dominant players (e.g., Clostridium) and the other degraded resistant dietary fibers later (e.g., Bifidobacterium, Bacteroides, Dysgonomonas).
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------

Electron transfer kinetics in biofilm anodes: conductive extracellular electron transfer
Abstract
This chapter reviews electron transfer kinetics from an electron donor to the anode in microbial electrochemical cells (MxCs), focusing on extracellular electron transfer (EET). Conductive EET would be responsible for high current density in biofilm anodes, and redox conduction, ohmic conduction, or combined conduction would occur in the biofilms. Metallic-like and redox conduction has been proved with pure culture of Geobacter biofilms, implying EET can be more complicated than we have thought in biofilm anodes. Interestingly, EET kinetics follows Ohmic conduction in mixed-culture electrically conductive biofilm anodes in which potential gradient for saturated current density is relatively small. As a consequence, MxCs can produce several hundreds of current density if intracellular electron transfer (biological kinetics) does not limit the current density. It seems essential to revisit biological parameter study to improve the current density, such as biofilm density, biofilm thickness, electrode configuration, and pretreatment of complex organics for anode-respiring bacteria.
-----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------