Introduction

I. Development of hydrocarbon-based sulfonated polymer electrolytes for proton exchange membrane water electrolyzers (PEMWEs) and proton exchange membrane fuel cells (PEMFCs)
With the growing demand for clean energy conversion systems, PEMWEs and PEMFCs are being developed as promising alternatives to fossil fuels as energy generation systems. Among the various components of PEMWEs and PEMFCs, proton exchange membranes (PEMs) are very important materials that determine their overall performance. The most widely used PEMs are perfluorinated sulfonic acid (PFSA) polymer membranes, such as Nafion®, which exhibit superior properties, including high proton conductivity and durability in a temperature range of 10–80 °C. However, these materials suffer several drawbacks, such as their high cost, complex synthetic protocols, and recent environmental and regulatory issues associated with polyfluoroalkyl substances.
To replace Nafion®-type PFSA PEMs, I have developed hydrocarbon-based sulfonated PEMs, which offer superior stability, high proton conductivity, and low-cost production. In particular, I have focused on developing technologies that can be applied practically in industry. I will continue to develop low-cost, high-performance PEMs for PEMWEs and PEMFCs, and devote considerable effort to leading the early commercialization of these energy conversion technologies.
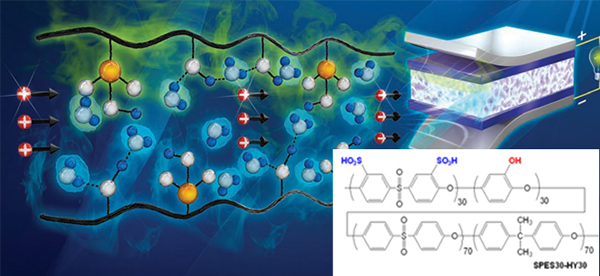
Hydrocarbon-based polymer electrolyte membranes for fuel cells and water electrolyzers
II. Development of base-tolerant anion exchange membranes for solid alkaline membrane water electrolyzers (SAMWEs) and solid alkaline membrane fuel cells (SAMFCs)
Recently, the development of water electrolyzers that produce hydrogen using renewable energy is being actively pursued. Studies on fuel cells capable of utilizing the hydrogen produced by water electrolyzers most efficiently are also being conducted. Hydrogen energy conversion technologies, such as electrolyzers and fuel cells, provide alternatives to fossil-fuel-based energy production, offering meaningful solutions to the current issues surrounding environmental impact and resource depletion.
Hydrogen energy conversion technologies based on proton (H+) conduction mechanisms, such as PEMFCs and PEMWEs, require the use of Nafion®-type PFSA electrolyte membranes, platinum group metal (PGM) catalysts, and oxidation-resistant porous transport media. As the supply of renewable energy increases, the demand for hydrogen production and utilization will also increase. Therefore, the high cost of the materials required for PEMFC and PEMWE manufacture will become a significant drawback. Many researchers are focusing on finding ways to lower the price of these materials, but they have not yet produced significant results.
Instead, I have been studying a technology that produces and utilizes hydrogen employing a mechanism that delivers hydroxide ions (OH-) rather than focusing on a proton conduction mechanism. SAMWEs and SAMFCs, which operate based on an OH- delivery mechanism, have the advantage of using low-cost electrolyte membranes and transition metal catalysts for energy conversion.
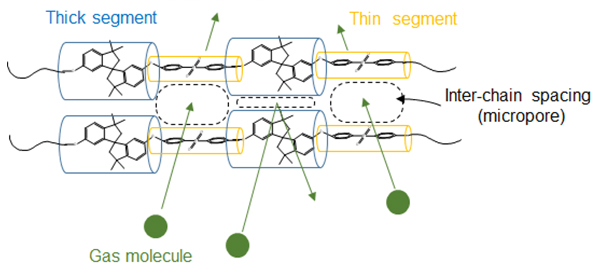
Modification of polymer structure for electrode binders in fuel cells and water electrolyzers
III. Development of self-humidifying dual exchange membrane fuel cells (DEMFCs)
The miniaturization of fuel cell systems is crucial for developing fuel cells for drones and unmanned aerial vehicles (UAVs). PEMFCs are generally used by these aircraft. In general, a humidifier is located next to the PEMFC because humidified fuel and air must be supplied to achieve high performances. Because the humidifier in fuel-cell-powered aircraft increases the weight and volume, it is necessary to develop a PEMFC that exhibits excellent performance without a humidifier. I developed a new-concept self-humidifying dual exchange membrane fuel cell (DEMFC) to overcome the limitations of the PEMFC.
The DEMFC was fabricated using sequentially aligned MEAs consisting of an anion exchange membrane (AEM) and a cation exchange membrane (CEM). The AEM and CEM MEA sections in the DEMFC are expected to be mutually humidified to enable non-humidified operation. On the anode side, dry hydrogen supplied to the AEM MEA section is electrochemically oxidized by hydroxide ions to produce water. The water generated at the anode of the AEM MEA section is transported through the gas diffusion medium (GDM) to humidify the anode and promote the HOR in the CEM MEA section. Meanwhile, on the cathode side, dry oxygen supplied to the CEM MEA section is electrochemically reduced by protons to produce water. Similarly, the water generated at the cathode of the CEM MEA section is supplied to the AEM MEA section to enhance the ORR. Currently, there are no fuel cells that generate water at the anode and cathode. For the first time, I have developed a new type of fuel cell that produces water at both the anode and cathode, allowing for the elimination of an external humidifier in a fuel cell system. The use of this technology is expected to facilitate fuel cell water management in the future. 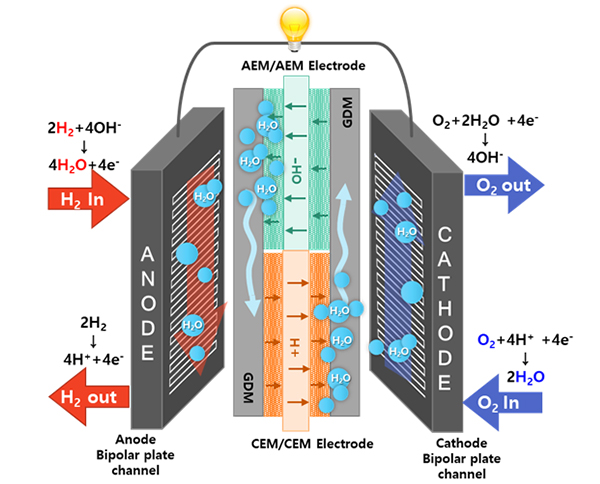
Dual exchange membrane fuel cell with sequentially aligned cation and anion exchange membranes for drones and UAVs